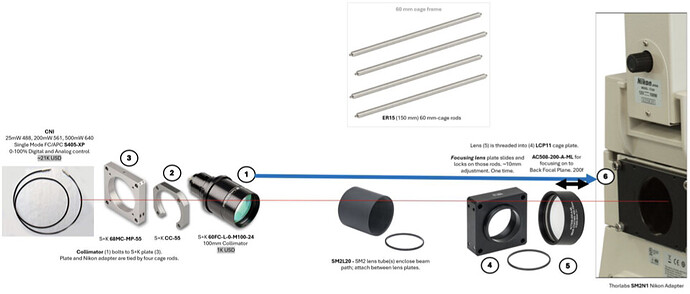
Dear μforum -
I have a Zeiss Axioobserver microscope, and a EMCCD camera, and wish to purchase a laser light source, and to set up a single molecule imaging system in my lab (I am a PhD student). However, I am ignorant of a good way to couple the lasers to the microscope.
In particular, I want to use the 100x, 1.4 numerical aperture objective, and to achieve a maximum power of 30 kW/cm^2 with a far-red laser of ~640nm, but to achieve much lower powers of 30 W/cm^2 with a 405nm, 488nm and 549nm laser. The illumination field should be at least 30 microns by 30 microns. I would like a Widefield, homogenous, square illumination field.
What do you recommend regarding laser engines and couplers, for a non-expert in optics, who is comfortable with reading the manual? A single component or very few components that I might purchase and easily assemble, in order to connect the high/low power lasers (uv,blue,red, and far-red), to the microscope, and to get Widefield illumination, would be ideal. Because the lab is an experimental biology lab, a coupling solution/part that couples the laser while completely encasing the laser beam is ideal.
I would be happy to hear also recommendations about which companies to consult with.
Thank you for your time -
Yossi
Weizmann Institute of Science